A team from Lei Dong and Junfeng Zhang, the School of Life Sciences, Nanjing University and the State Key Laboratory of Pharmaceutical Biotechnology, and Chunming Wang, University of Macau, published online an original research paper entitled Reprogramming the Spleen into a Functioning Liver in vivo [1] in Gut on 7 January 2022. This study transformed the spleen to perform liver functions in mice through spleen tissue remodeling and direct reprogramming, without transplanting cells or tissue from another body.
At least two million people die each year due to liver diseases. Orthotopic liver transplantation is the most effective treatment for end-stage liver disease. However, the dearth of donor organs is a critical challenge worldwide. It’s vital to develop alternatives to liver transplantation. Some simple tissues and organs have been constructed through tissue engineering methods. But it cannot rebuild the rich network of blood vessels. Instead of engineering an organ for transplantation, the team chose to directly transform an existing organ - the spleen - into a 'new' organ that fulfils the liver's function.
In June 2020, the team published a study in Science Advances that had transformed murine spleen into a liver-functioning organ[2]. Firstly, the spleen was injected with an active biological reagent to reshape its microenvironment, increase matrix content, reduce immune rejection and enrich blood vessels. Secondly, hepatocytes from different species origin were transplanted into the remodeled spleen. And Transplanted hepatocytes grew, proliferate and functioned well. This study addressed the growth, integration, and functionalization of regenerated liver, but the inherent problems of immune rejection and seed cell sources has not been solved. The new study, published in Gut, exploited direct reprogramming technique to turn auto-fibroblasts directly into functional hepatocytes, theoretically eliminating the problems of immune rejection and cell donors and essentially allowing the spleen to grow its own liver tissue.
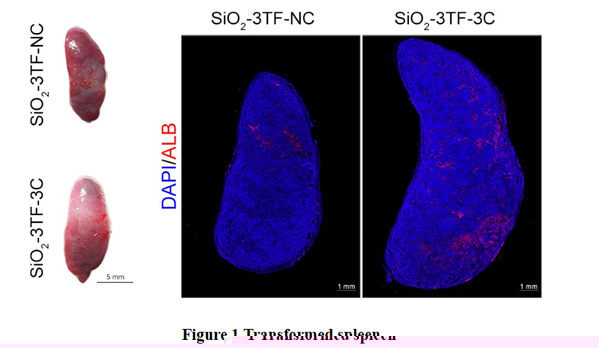
To facilitate continuous injection and observation of the remodeling spleen, the spleen of a mouse was surgically moved from the inner abdomen to a subcutaneous site. Then, silicon oxide (SiO2) nanoparticles were injected into the translocated spleen to promote the proliferation of fibroblasts, laying the foundation for subsequent reprogramming and liver regeneration. Next, the team delivered Foxa3, Gata4 and Hnf1a into SiO2-remodeled spleen by a lentiviral vector to reprogram the splenic activated fibroblasts into a functional hepatocytes (iHeps) in situ. Finally, the spleen was further transported tumor necrosis factor (TNF-α), epidermal growth factor (EGF) and hepatocyte growth factor (HGF) (combination referred to as 3C) to realize the proliferation of iHeps in vivo. And the proportion of iHeps in the reprogrammed spleen reached about 8 × 106. Having had grown for 6 months in the spleen, these iHeps exert
physiological functions of the liver (Figure 1).
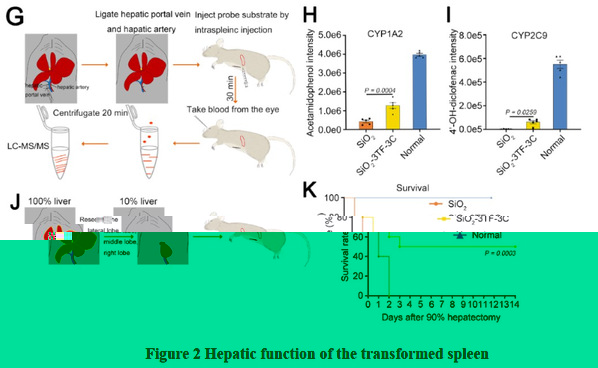
More importantly, in 90% hepatectomy induced acute liver failure, the survival rate of mice with transformed spleen was 50%, while the control mice all died, indicating that transformed spleen possessed perfect liver function (Figure 2).
Different from all existing means such as whole-organ transplantation, tissue engineering and cell transplantation, the study demonstrated in vivo restoration of liver functions in the murine spleen. Notably, this approach does not transplant any exogenous cells or organs, which might cause immune rejection or unexpected safety issues. It provides a new brand strategy for functional reconstruction of large tissue and therapy of end-stage liver disease.
Prof. Lei Dong and Junfeng Zhang of Nanjing University and Prof. Chunming Wang of University of Macau are the corresponding authors of the article. Dr. Chunyan Liu from Nanjing University's School of Life Sciences and Dr. Lintao Wang, a Haojiang scholar at the University of Macau, are co-first authors of the article. The study was funded by the National Natural Science Foundation of China (NSFC) and the Science and Technology Development Fund (FDCT) (Dong Lei from the Mainland, Wang Chunming from Macao).
Article link:
1. https://gut.bmj.com/content/early/2022/01/06/gutjnl-2021-325018
2. https://doi.org/10.1126/sciadv.aaz9974
