Nuclear receptor PPAR(Peroxisome proliferator-activated receptor gamma) is a key molecular sensor for extracellular nutrition and energy. It is also the controller/plant for regulating cellular metabolic flux and cell fate determination. In recent years, Prof. Pingping Shen's group has made a series of progress in the research of deciphering the assembling of PPAR post-translational modification associated protein machine, and identifying the related key metabolic regulators in the protein machine. These research findings have been published in Signal Transduct Target Ther, Nat Commun and Adv Sci etc. Based on these crucial achievements, Shen's group has developed the novel metabolic reprogramming strategies for cell engineering, and further constructing the related cell therapy regimens.
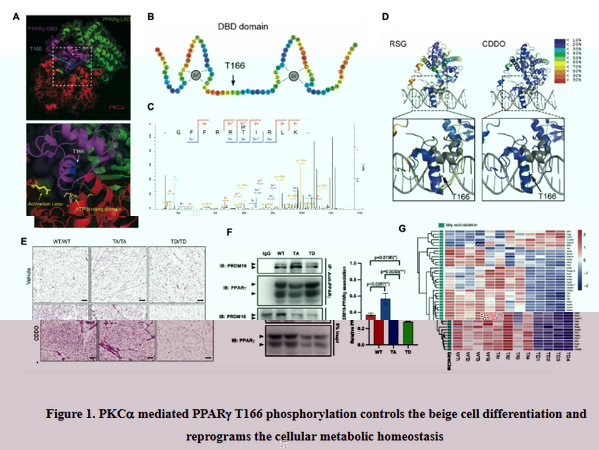
Recently, with the collaboration of Prof. Xiaokun Li (Academician of Chinese Academy of Engineering) in Wenzhou medical university, Shen's group has made an important breakthrough in PPAR biology—Discovering an uncharacterized post-translational modification pattern (T166 phosphorylation) in PPAR DNA binding domain (DBD), and uncovering its entangled function in switching beige cell differentiation (Figure 1). Further molecular mechanism investigation proves that T166 is located between two zinc finger structures in DBD. When the T166 is phosphorylated by PKC, the fine-tuning of DBD conformation changes the allosteric communication between the LBD and DBD, influences the binding affinity of transcriptional coactivators to PPAR ligand binding domain (LBD), which further regulates the PPAR[符号]-mediated transactivation of beige cell-related gene expression in white adipose tissue (WAT) and thus impacts on beige cell differentiation. Based on the important role of PPAR T166 phosphorylation in beige cell metabolic reprogramming, the research group designs the associated genetic/chemical intervention strategies targeting PKC-PPAR axis for highly inducing beige cell biogenesis in WAT, and improving the metabolic dysfunctions. This study not only provides an advanced molecular insight into the basic biological function of PPAR in adipose biology, but also offers a novel therapeutic avenue for the treatment of metabolic diseases.
This work was published in Cell death & Differentiation entitled by “Blockage of PPARγ T166 phosphorylation enhances the inducibility of beige adipocytes and improves metabolic dysfunctions” (DOI:10.1038/s41418-022-01077-x). The first author of this paper was Dr. Nanfei Yang. Prof. Pingping Shen and Prof. Xiaokun Li were the corresponding authors. Prof. Jianguo Li (Peking University) ,Prof. Hu Zhou (Chinese Academy of Sciences), Prof. Wei Yang (UCLA) and Prof. Zhifeng Huang (Wenzhou medical University) participated in this work. This work was financed by grants from the National Key Research and Development Plan (2017YFA0506000), Guangdong Basic and Applied Basic Research Foundation (2021B1515120016). We are grateful to Tissue Gnostics Asia Pacific Ltd for their technical support.
https://doi.org/10.1038/s41418-022-01077-x
