Recently, Professor Zhu Hailiang′s team from the School of Life Sciences of Nanjing University and their collaborators have made new progress in the treatment of central nervous system tumors with transferrin nanomaterials.
The tumor microenvironment (TME) has different physical and chemical characteristics from normal physiological conditions, such as hypoxia, low pH, high GSH and high vascular permeability. Among them, hypoxic environment is closely related to tumor growth, metastasis and prognosis. The uncontrolled proliferation and abnormal development of blood vessels inside the tumor determine the high metabolic characteristics of the tumor. The high metabolic characteristics of tumor cells cause hypoxic microenvironment inside tumor cells, which has a screening effect on tumor cells, further increasing the malignancy of tumor, leading to tumor cells insensitive to chemotherapy drugs or radiotherapy. Based on the previous development of nanocarrier platform for solving TME hypoxia problem (ACS Appl. Mater. Interfaces 2020, 12, 24662-24674), Professor Zhu Hailiang′s team from the School of Life Sciences of Nanjing University constructed for the first time an in situ glioma-targeted transferrin-mediated endogenous stimulus-responsive multifunctional nanocarrier platform (Figure 1) according to the homologous recognition ability of transferrin and its receptor. The platform solves the problems of drug crossing blood-brain barrier, targeted slow-release drug, imaging tracing and tumor hypoxia, significantly inhibits the growth of various malignant tumors including central nervous system (CNS) tumors, alleviates the refractory hypoxia problem in solid tumor microenvironment, realizes the synergistic enhancement of photodynamic therapy combined with chemotherapy effect, and provides a new strategy for the treatment of malignant tumor.
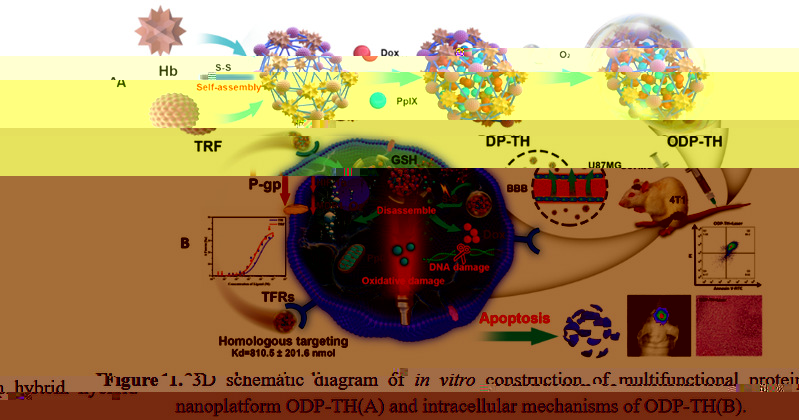
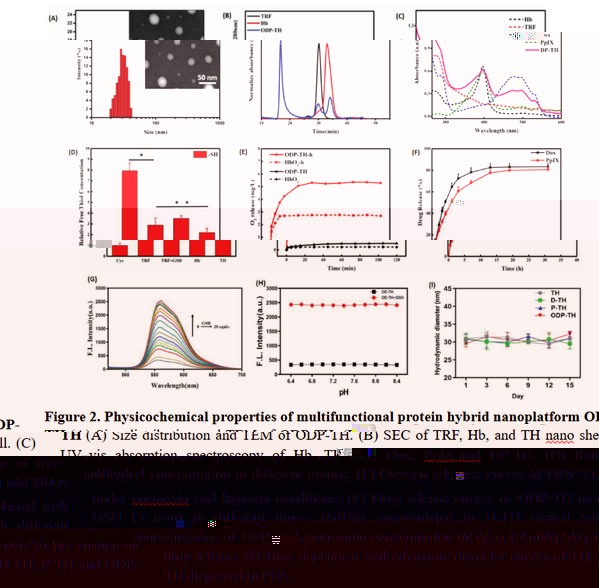
The chemotherapeutic drug Dox and the photosensitizer PpIX were encapsulated in transferrin and hemoglobin through the self-assembly method of disulfide bond reconstruction, and oxygen was integrated in vitro to construct a glioma with both oxygen-carrying ability and precise targeting function of in situ glioma Hybrid protein nano drug delivery platform ODP-TH. On the one hand, the transferrin drug carrier can specifically recognize and bind to the transferrin receptor overexpressed on the cell surface of tumors and brain endothelial cells, cross the blood-brain barrier mediated by the transferrin receptor, and selectively enrich to the tumor cells of the central nervous system, the encapsulated drug and the carried oxygen are released rapidly and continuously in the tumor microenvironment with low oxygen and high GSH concentration. On the other hand, the study of multifunctional nano-drug delivery system mediated by oxygen-supplying transferrin is helpful for understanding the disease mechanism caused by hypoxia in the tumor microenvironment, and the established system has good biological safety, strong targeting, rapid response and Synergistic anti-tumor oxygen-supply drug nano-delivery platform method, solves the expression disorder of various oxygen-related genes (MDR1, HIF-1α and P-gp) caused by hypoxia, reduces the efflux of chemotherapy drugs, and alleviates common resistance in chemotherapy Adverse drug phenomenon has far-reaching practical application value. The smaller nanomaterial particle size (30.2±1.2 nm) maximizes the oxygen carrying on the surface of ODP-TH, enabling it to complete the free conversion from oxygenated conformation to deoxygenated conformation within 30 min in a low pO2 environment. Strong disulfide bond rivets make ODP-TH stable in form and function in non-TME. Once entering the reduced GSH-enriched TME, its excellent GSH response mechanism can ensure the rapid disintegration of nanomaterials, ensuring that the synergistic therapeutic function of the entire platform can quickly work in a specific cancer focus area (Figure 2).
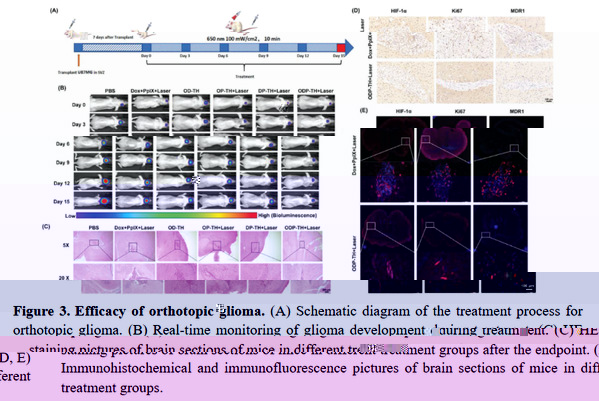
The team also constructed an in-situ glioma animal model, and systematically evaluated the function and pharmaceutical characteristics of the nano-platform targeting glioma across the blood-brain barrier using liquid chromatography-mass spectrometry, tumor immunity, protein purification, cell migration detection, multimodal imaging, and other technologies. The platform realizes the synergistic enhancement of multi-dimensional photodynamic therapy combined with chemotherapy through targeted transport based on oxygen (Figure 3), which not only provides a new method for the complete cure of glioma in the future, but also provides new ideas for the mutual integration and development of life science, medicine, chemistry and materials science and other disciplines.
The article titled “Multifunctional Protein Hybrid Nanoplatform for Synergetic Photodynamic Chemotherapy of Malignant Carcinoma by Homologous Targeting Combined with Oxygen Transport” was published in Advanced Science 2022, 2203742. The first author of this article is Wu Songyu, a doctoral student of Nanjing University School of Life Sciences in 2018. The corresponding authors of this article are Professor Zhu Hailiang and Associate Researcher Wang Zhongchang of Nanjing University School of Life Sciences, Associate Professor Zou Meijuan of Nanjing Medical University and Associate Researcher Duan Yongtao of Zhengzhou Children′s Hospital affiliated to Zhengzhou University. The research work was supported by the National Natural Science Foundation of China, China Postdoctoral Science Foundation and other projects. The online link of the paper is: https://doi.org/10.1002/advs.202203742.
