Dysregulation of RBPs (RNA Binding Proteins) can lead to many diseases. Regulating RBP function is emerging as a promising therapeutic strategy. However, targeting RBPs with small molecules remains a challenge because most RBPs are considered undruggable due to lacking of well-defined binding pockets. Recently, our team reported that RNA binding protein CELF1 plays a crucial role in numerous fibrotic diseases and is determined as a novel therapeutic target. (Nat Commun 2016; Toxicol Appl Pharmacol 2021).
To discover CELF1 inhibitors, we proposed a strategy by virtual screening compounds that mimicking the CELF1-Guanine interaction. For the first time, compound 27 was identified as a hit compound to disrupt CELF1-RNA interaction by competing with RNA for binding to CELF1. Next, we found that compound 27 could enhance antifibrotic signal IFN-γ and inhibit hepatic stellate cell activation in vitro and iv vivo (Figure 1). Furthermore, we found compound 841, a derivative of compound 27 based on the amino iso-quinoline pharmacophore, posed a more potent therapeutic activity. These compounds have been patented (ZL202111009467.9; ZL202210029098.8) and may bring new perspectives and novel approaches to treat liver fibrosis and other CELF1 related diseases.
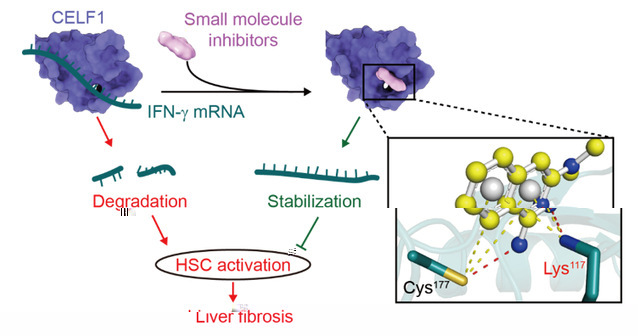
Figure 1. Disruption of CELF1-RNA binding by small molecule inhibitors for controlling HSC activation.
This study has been published in the Nucleic Acids Research, titled with Small molecule targeting CELF1 RNA binding activity to control HSC activation and live fibrosis. The link to the paper is: https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkac139/6541020。

Professor Xingxin Wu and professor Qiang Xu (School of Life Sciences, Nanjing University and State Key Laboratory of Pharmaceutical Biotechnology) are the co-corresponding authors of this paper. Yang Tan, Xueqing Sun and Yizhu Xu (School of Life Sciences, Nanjing University) are the co-first authors of this paper. This study was funded by the National Natural Science Foundation of China, the National Key R&D Project Fund, the research business fees of central universities and the China Postdoctoral Science Foundation, as well as assistance and support from cooperative laboratories.
